How is an Oil Droplet and a Soccer Ball Similar?
New metamaterial research finds that guest molecules arrange themselves in a fixed pattern on the surface of a liquid droplet
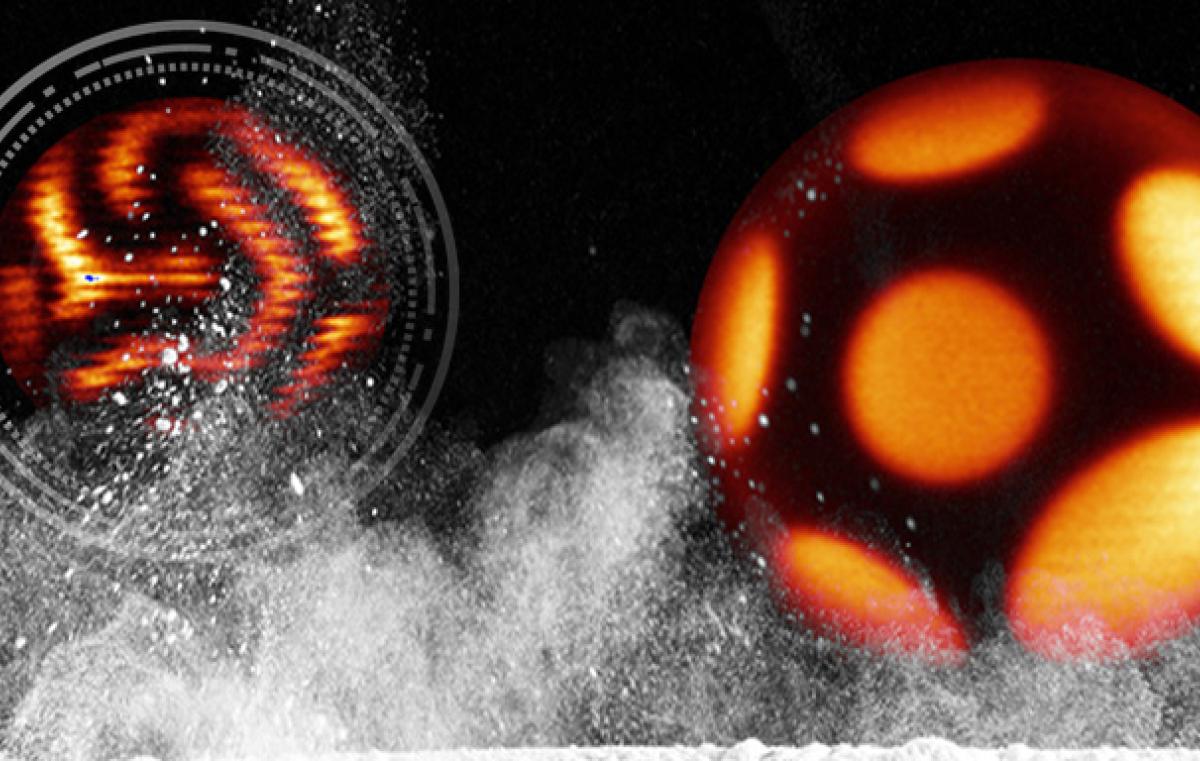
A study conducted at Bar-Ilan University has demonstrated that a guest substance can organize itself in a fixed pattern on the surface of a fatty liquid droplet. The discovery may have far-reaching applications in the hot, promising field of metamaterials for use in medicine and industry.
A team of physicists and chemists from Bar-Ilan University has developed a method that makes it possible to control the position of guest molecules on the surface of a spherical liquid droplet. The molecules independently pattern themselves in 12 precise positions on the droplet. If we envision the liquid droplet as a soccer ball, these 12 locations are exactly in the centers of the black pentagons of the ball's surface. The process is controlled by the temperature of the model and is not limited to a specific type of guest molecule: any surface-active molecule, i.e., a molecule adsorbed to the surface of the droplet, will behave similarly.
At low temperatures, the guest molecules line up on the droplet’s surface at a maximum distance from each other (imagine the black pentagons on the soccer ball’s surface shrinking). At high temperatures, the spots of the guest molecules spread across the droplet in a specific pattern. The researchers were surprised to find that the same pattern repeated itself in all the millions of droplets in each sample.
In the experiment, researchers used a fatty liquid for the droplets and fluorescent molecules as guest molecules since they can be tracked. The basic mechanism they deciphered to explain the phenomenon may play an important role in biological systems: from the envelopes of viruses to the location of proteins on the living cell membrane. In the fields of micromaterials and nanomaterials, this phenomenon can be used to produce smart connections that allow droplets to connect together, forming supradroplet structures, and to precisely create metamaterials – engineered materials with a unique microscopic structure that does not exist in nature and gives them unique properties on demand.
The new method of placing molecules on liquid droplets also has implications for almost every field where emulsions are used: the food industry, water purification, oil industries, nanomedicine, and more. For example, some vaccines use liquid droplets "decorated" with antigen molecules. The location and spatial orientation of the antigen molecules affect the vaccine’s efficacy. The method developed by the researchers makes it possible to control these parameters to improve the effectiveness of vaccines, so that they can be given in lower doses, reducing the risks of harmful side effects. It is also possible to think of "smart" droplets on which you can load a drug cargo, which will be released when the droplet reaches its destination. In the food industry, this novel method may be used to change texture, taste, shelf life, digestion process, health effects, and more. Prof. Eli Sloutskin, one of the leaders of the research team, points out that all these directions are very promising, but have not yet been fully explored.
Prof. Sloutskin uses the soccer model to describe the mechanism that explains the self-patterning phenomena of the guest molecules on the surface of the liquid droplet: at certain temperatures, the oil molecules organize into a crystalline monolayer, which covers the surface of the droplets. The formation of such a crystalline monolayer, although counterintuitive, has previously been observed in many oils. These crystalline layers have a hexagonal structure, but it is not possible to produce a closed layer made only of hexagons: for the layer to be closed, you need to use exactly 12 pentagons, in addition to the hexagons. This is the reason why there are 12 (black) pentagons in a soccer ball, while all the remaining (white) parts are hexagons. Similarly, in the crystalline layers covering the surface of the droplets, there are exactly 12 pentagonal "defects": molecules which have five nearest neighbors, instead of six. The defects in the crystal cause stress inside the crystal. In order to alleviate this stress, the 12 defects are pushed away from each other, as far as possible. Thus, the 12 defects position themselves at spots which correspond to the centers of the 12 pentagons of the soccer ball. If we now introduce guest molecules into the system, they will attach to the positions of the defects, to compensate to some extent for crystal’s deformations at these locations and decrease the stress. Thus, it turns out that the guest molecules will be found in 12 focal points on the spherical droplet, with these foci being as far apart as possible.
The study, co-authored by Prof. Eli Sloutskin, Dr. Alexander Butenko and Prof. Moshe Deutsch (of the Department of Physics); and Prof. Yitzhak Mastai and Dr. Subhomoy Das (of the Department of Chemistry) at Bar-Ilan University, was published in the Nature Physics journal.